Chimeric antigen receptor T-cell (CAR-T) therapy has made breakthroughs in the field of tumor treatment, and the optimization of the downstream purification process of lentiviral vector (LV), as the core raw material of the gene delivery system, is directly related to the safety and economy of the treatment. In this paper, we systematically review the technological progress of the three major stages of the downstream purification process of LV - clarification filtration, purification and purification sterilization, focusing on the application strategy of tangential flow filtration (TFF), analyzing the influence of membrane material selection and process parameter control on the activity and recovery of LV, and proposing the challenges and future research directions of the large-scale production of LV. We also propose the challenges and future research directions in the large-scale production of LV.
The importance of CAR-T therapy and lentiviral vectors
CAR-T therapy achieves precise anti-tumor effects by genetically engineering patient T cells, and its clinical efficacy has achieved significant breakthroughs in hematologic malignancies. However, the production process of CAR-T cells is complex, in which the cost of lentiviral vector-mediated gene transduction accounts for as much as 30%-50%, and the activity and purity of LV directly affect the function and safety of CAR-T cells. Therefore, the construction of an efficient, stable and GMP-compliant LV downstream purification process has become a core issue to promote the industrialization of CAR-T therapy.
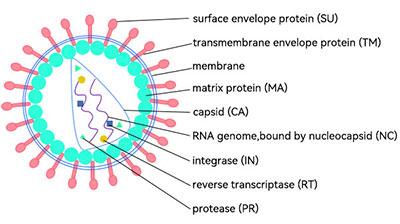
Three steps in the downstream purification of LV
LV activity is affected by temperature, pH, salt concentration, osmotic pressure, and shear during the process, so extra attention needs to be paid to the effect of these conditions on the activity of lentiviral vectors during downstream purification.
1. Clarification and filtration
Clarification and filtration are designed to remove cellular debris and large impurities (>1 μm) from cell culture fluids. The current mainstream process combines low-speed centrifugation (<5000×g) with deep filtration technology, which reduces the risk of filter clogging through a staggered-flow design, and is particularly suitable for continuous processing at pilot scale and above. For example, the use of polyethersulfone (PES) deep filtration membranes with a pore size of 0.45-1.2 μm can efficiently retain particulate contaminants and reduce the load of host cell proteins (HCPs) by more than 80%, thus laying a high-quality foundation for the subsequent purification stage.
1. Clarification and filtration
Clarification and filtration are designed to remove cellular debris and large impurities (>1 μm) from cell culture fluids. The current mainstream process combines low-speed centrifugation (<5000×g) with deep filtration technology, which reduces the risk of filter clogging through a staggered-flow design, and is particularly suitable for continuous processing at pilot scale and above. For example, the use of polyethersulfone (PES) deep filtration membranes with a pore size of 0.45-1.2 μm can efficiently retain particulate contaminants and reduce the load of host cell proteins (HCPs) by more than 80%, thus laying a high-quality foundation for the subsequent purification stage.
2. Purification
The main strategies applicable to the moderate purification of LV include chromatography and tangential flow filtration. Ultrafiltration diafiltration (UFDF) in tangential flow filtration (TFF) is a key step in the large-scale purification and concentration of lentiviral vectors (LV), as well as in buffer exchanget.
During the UFDF process, virus particles are concentrated while impurities with molecular weights smaller than the membrane pore size (e.g. DNA) are effectively removed with the permeate. From the process flow point of view, TFF can be applied either in the post-clarification stage to reduce the volume of material in the subsequent chromatography step through concentration, or between moderate purification and fine purification to adjust the pH and conductivity of the sample through buffer exchange, thus creating favorable conditions for subsequent chromatography operations.
Since LV is easily inactivated, the process requires strict control of parameters such as shear force, flux, transmembrane pressure and operation time, as well as selection of appropriate membrane materials and pore sizes to ensure LV activity. Membrane materials suitable for LV purification include polyethersulfone (PES) and regenerated cellulose (RC). In addition, the choice of membrane pore size is critical: larger pore sizes result in higher impurity removal, but may result in lower LV recovery. Therefore, the most appropriate membrane material and pore size need to be selected based on specific process conditions.
3. Purification and sterilization
The main strategies applicable to LV purification are SEC (size exclusion chromatography) as well as TFF (tangential flow filtration). Further concentration by TFF is available for groups requiring higher concentrations of virus. However, high shear (>5000 s-¹) and transmembrane pressure (TMP >1.5 bar) can lead to rupture of the LV capsid, and the flux needs to be maintained at 20-40 LMH by dynamic pressure control to maintain activity. Finally, aseptic filtration is performed, usually with a 0.22 μm filter membrane for filling.
The main strategies applicable to the moderate purification of LV include chromatography and tangential flow filtration. Ultrafiltration diafiltration (UFDF) in tangential flow filtration (TFF) is a key step in the large-scale purification and concentration of lentiviral vectors (LV), as well as in buffer exchanget.
During the UFDF process, virus particles are concentrated while impurities with molecular weights smaller than the membrane pore size (e.g. DNA) are effectively removed with the permeate. From the process flow point of view, TFF can be applied either in the post-clarification stage to reduce the volume of material in the subsequent chromatography step through concentration, or between moderate purification and fine purification to adjust the pH and conductivity of the sample through buffer exchange, thus creating favorable conditions for subsequent chromatography operations.
Since LV is easily inactivated, the process requires strict control of parameters such as shear force, flux, transmembrane pressure and operation time, as well as selection of appropriate membrane materials and pore sizes to ensure LV activity. Membrane materials suitable for LV purification include polyethersulfone (PES) and regenerated cellulose (RC). In addition, the choice of membrane pore size is critical: larger pore sizes result in higher impurity removal, but may result in lower LV recovery. Therefore, the most appropriate membrane material and pore size need to be selected based on specific process conditions.
3. Purification and sterilization
The main strategies applicable to LV purification are SEC (size exclusion chromatography) as well as TFF (tangential flow filtration). Further concentration by TFF is available for groups requiring higher concentrations of virus. However, high shear (>5000 s-¹) and transmembrane pressure (TMP >1.5 bar) can lead to rupture of the LV capsid, and the flux needs to be maintained at 20-40 LMH by dynamic pressure control to maintain activity. Finally, aseptic filtration is performed, usually with a 0.22 μm filter membrane for filling.
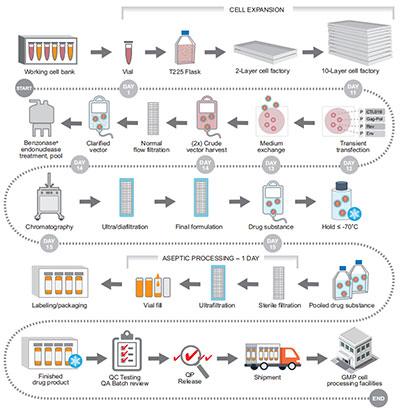
Image source:Molecular Therapy
Challenges and Future Developments
Although significant progress has been made in LV purification technology, the following problems exist:
(1) batch-to-batch consistency differences due to membrane contamination;
(2) High capital investment in ultracentrifugation and chromatography equipment;
(3) The cost of cold chain transportation due to the lack of LV stability in vitro.
To further enhance the accessibility and economics of LV production, future research could focus on:
(1) Development of new biomimetic membrane materials (e.g. graphene oxide composite membranes)
(2) Integration of continuous flow purification process
(3) Breakthrough in lyophilized formulation technology
Downstream purification of lentiviral vectors (LV) is a core aspect of CAR-T therapy industrialization, and the application of tangential flow filtration (TFF) significantly improves the purity and recovery of LVs. However, membrane contamination, process stability and cold chain dependence are still major challenges. In the future, through the development of new materials, continuous flow process optimization and lyophilization technological innovation, it is expected to further break through the technological bottleneck and promote CAR-T therapy from the laboratory to wider clinical applications.
Although significant progress has been made in LV purification technology, the following problems exist:
(1) batch-to-batch consistency differences due to membrane contamination;
(2) High capital investment in ultracentrifugation and chromatography equipment;
(3) The cost of cold chain transportation due to the lack of LV stability in vitro.
To further enhance the accessibility and economics of LV production, future research could focus on:
(1) Development of new biomimetic membrane materials (e.g. graphene oxide composite membranes)
(2) Integration of continuous flow purification process
(3) Breakthrough in lyophilized formulation technology
Downstream purification of lentiviral vectors (LV) is a core aspect of CAR-T therapy industrialization, and the application of tangential flow filtration (TFF) significantly improves the purity and recovery of LVs. However, membrane contamination, process stability and cold chain dependence are still major challenges. In the future, through the development of new materials, continuous flow process optimization and lyophilization technological innovation, it is expected to further break through the technological bottleneck and promote CAR-T therapy from the laboratory to wider clinical applications.








