At the critical juncture of scaling up biopharmaceutical production for commercialization, the industry faces a fundamental challenge: how to establish a sustainable equilibrium between efficiency and robustness? As the flexibility of single-use systems reaches its ceiling for large-scale manufacturing, and cost control and supply chain security become strategic imperatives, we must re-examine the foundational logic of production platforms.
HOLVES now officially launches the Omini Cell510 All-Purpose Bioreactor, an intelligent cell culture platform engineered for strategic production. It transcends traditional approaches by systematically redefining three core values—robustness, cost-effectiveness, and autonomy—through environmental predictability, full-cycle economic efficiency, operational autonomy, and scenario-specific adaptability.
HOLVES now officially launches the Omini Cell510 All-Purpose Bioreactor, an intelligent cell culture platform engineered for strategic production. It transcends traditional approaches by systematically redefining three core values—robustness, cost-effectiveness, and autonomy—through environmental predictability, full-cycle economic efficiency, operational autonomy, and scenario-specific adaptability.
-
Reliability: Born of precision control, perfected through process reproducibility
In large-scale production, the cost of process fluctuations far exceeds material losses. Robustness stems from precise control over every physical and biochemical parameter. Through deep integration of hardware and control systems, the Omini Cell510 transforms “stability” from a concept into a quantifiable engineering practice.
1. Computational Fluid Dynamics Optimization: The system incorporates a CFD-optimized combination of agitation and aeration. Its low-shear-force field design not only facilitates efficient transfer of nutrients, dissolved oxygen(DO), and metabolites but also significantly reduces mechanical damage to cells—particularly shear-sensitive cell lines—creating ideal physical conditions for high cell viability and efficient expression.
2. Automated Sterility Assurance: Standard one-touch fully automated SIP/CIP functionality. Utilizing four-channel simultaneous steam sterilization at 121°C for 30 minutes, validated through professional pressure retention testing, it establishes a sterility barrier surpassing manual methods.
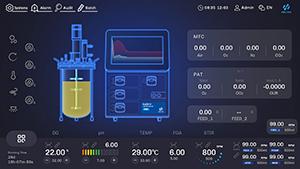
3. Data Traceability and Process Anchoring: The HF-Control V3.0 Holographic Intelligent Control System enables millisecond-level monitoring and closed-loop control of critical parameters. Its open industrial data interfaces seamlessly integrate with upper-level management systems, laying the foundation for a digital, traceable production system.
1. Computational Fluid Dynamics Optimization: The system incorporates a CFD-optimized combination of agitation and aeration. Its low-shear-force field design not only facilitates efficient transfer of nutrients, dissolved oxygen(DO), and metabolites but also significantly reduces mechanical damage to cells—particularly shear-sensitive cell lines—creating ideal physical conditions for high cell viability and efficient expression.
2. Automated Sterility Assurance: Standard one-touch fully automated SIP/CIP functionality. Utilizing four-channel simultaneous steam sterilization at 121°C for 30 minutes, validated through professional pressure retention testing, it establishes a sterility barrier surpassing manual methods.
3. Data Traceability and Process Anchoring: The HF-Control V3.0 Holographic Intelligent Control System enables millisecond-level monitoring and closed-loop control of critical parameters. Its open industrial data interfaces seamlessly integrate with upper-level management systems, laying the foundation for a digital, traceable production system.
-
Economical: Beyond initial investment, optimizing the entire lifecycle
The true cost of production must be assessed within the context of the entire lifecycle. The economic rationale behind the Omini Cell510 is precisely based on this principle.
1. Capital Investment: Constructed from 316L stainless steel with a modular design, the equipment boasts a lifespan spanning decades. Batch costs decrease exponentially with increased scale.
2. Flexible Production Capacity and Low Marginal Costs: Supports linear scaling from 30L to 2000L without requiring core hardware replacement. Enterprises can make precise investments based on budget and process requirements, avoiding resource wastage.
1. Capital Investment: Constructed from 316L stainless steel with a modular design, the equipment boasts a lifespan spanning decades. Batch costs decrease exponentially with increased scale.
2. Flexible Production Capacity and Low Marginal Costs: Supports linear scaling from 30L to 2000L without requiring core hardware replacement. Enterprises can make precise investments based on budget and process requirements, avoiding resource wastage.
3. Localized Services Reduce Hidden Costs: Leveraging local supply chains and technical support networks, we minimize downtime risks and operational costs from parts supply to responsive maintenance.
-
Autonomy: Taking control from equipment to process
Supply chain volatility and technological dependency have become latent risks in biomanufacturing. Omini Cell510 empowers enterprises to regain control over production through systematic design.
1. Supply Chain Security: Core components utilize standard 316L stainless steel, while non-contact material/liquid sections employ 304 stainless steel. Sealing components such as gaskets are made from pharmaceutical-grade polymer materials. The supply chain is fully autonomous and controllable, ensuring production safety from the source.
1. Supply Chain Security: Core components utilize standard 316L stainless steel, while non-contact material/liquid sections employ 304 stainless steel. Sealing components such as gaskets are made from pharmaceutical-grade polymer materials. The supply chain is fully autonomous and controllable, ensuring production safety from the source.
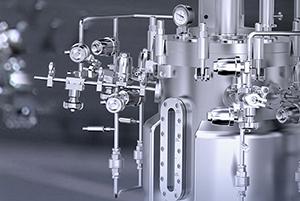
2. Flexible Process Path Compatibility: Compatible with both single-use aseptic sampling systems and traditional stainless steel piping. Users can flexibly switch process modes based on product characteristics without being constrained by pre-set equipment paths.
3. Sustainability of Technological Iteration: The modular architecture incorporates interfaces for PAT detection and cascading expansion, ensuring the equipment can be upgraded to meet evolving process requirements and facilitating long-term investment.
Omini Cell510 demonstrates critical value across multiple cutting-edge fields. Whether in the large-scale production of monoclonal antibodies (mAb) demanding batch-to-batch consistency, mRNA vaccines and viral vector manufacturing requiring stringent shear force and sterile conditions, or complex culture systems for cell and gene therapies, it serves as a reliable cornerstone for industrialization through precise control, flexible configuration, and robust data traceability capabilities.
3. Sustainability of Technological Iteration: The modular architecture incorporates interfaces for PAT detection and cascading expansion, ensuring the equipment can be upgraded to meet evolving process requirements and facilitating long-term investment.
Omini Cell510 demonstrates critical value across multiple cutting-edge fields. Whether in the large-scale production of monoclonal antibodies (mAb) demanding batch-to-batch consistency, mRNA vaccines and viral vector manufacturing requiring stringent shear force and sterile conditions, or complex culture systems for cell and gene therapies, it serves as a reliable cornerstone for industrialization through precise control, flexible configuration, and robust data traceability capabilities.
The launch of the Omini Cell510 is more than just the release of a high-performance device. It marks a return to long-term value, deep craftsmanship, and strategic security amid an industry trend chasing short-term efficiency. It is designed to be the most reliable element behind your R&D—silent, robust, and consistently dependable.
If your process is poised to make the critical leap from “successful experimentation” to “commercial success,” HOLVES invites you to explore the possibilities of this strategic-level solution together.
If your process is poised to make the critical leap from “successful experimentation” to “commercial success,” HOLVES invites you to explore the possibilities of this strategic-level solution together.