When cell culture processes complete early-stage development and enter the critical phase of pilot-scale and large-scale production, practical challenges initially masked by convenience begin to surface. The scaling-up process presents numerous obstacles, testing both process stability and the foresight of decision-makers.
At this critical juncture, we face a choice: continue relying on the flexibility of rapid deployment, or pivot toward pursuing deep, long-term development. If we choose the latter, the next question becomes more pressing: Does opting for stainless steel bioreactors mean sacrificing operational agility and intelligent evolution?
At this critical juncture, we face a choice: continue relying on the flexibility of rapid deployment, or pivot toward pursuing deep, long-term development. If we choose the latter, the next question becomes more pressing: Does opting for stainless steel bioreactors mean sacrificing operational agility and intelligent evolution?
In large-scale production, the consequences of process fluctuations are significantly magnified. Within single-use systems, “consistency” is often constrained by batch-to-batch variations in consumables and limited process adjustment ranges. As scale increases, physical limitations emerge in mixing efficiency, mass transfer uniformity, and parameter control precision. These limitations may lead to unpredictability in cell growth and metabolism, as well as potential fluctuations in product quality.
The foundation of reliability is often built upon the certainty of physical structures and the controllability of process parameters. Modern stainless steel bioreactors provide cells with a highly uniform and stable growth environment through their robust physical structures and precise fluid dynamics design. Their core advantage lies in the precise control of process parameters. From the shear force distribution of the agitator to the oxygen transfer efficiency, every variable is optimized and standardized based on engineering principles, thereby minimizing process fluctuations.
This stability directly translates into certainty in the production process, reducing time and material losses caused by batch failures, and establishing a reliable foundation for continuous, efficient commercial production.
The cost model for single-use systems is clear and straightforward, yet their true economic value often only becomes fully apparent after scaling up production. The continuous consumption of bioreactor bags, sensors, and tubing creates an invisible stream of operational expenditure.
When we shift our perspective from “purchase cost” to “total cost of ownership,” a different economic landscape unfolds.
The foundation of reliability is often built upon the certainty of physical structures and the controllability of process parameters. Modern stainless steel bioreactors provide cells with a highly uniform and stable growth environment through their robust physical structures and precise fluid dynamics design. Their core advantage lies in the precise control of process parameters. From the shear force distribution of the agitator to the oxygen transfer efficiency, every variable is optimized and standardized based on engineering principles, thereby minimizing process fluctuations.
This stability directly translates into certainty in the production process, reducing time and material losses caused by batch failures, and establishing a reliable foundation for continuous, efficient commercial production.
The cost model for single-use systems is clear and straightforward, yet their true economic value often only becomes fully apparent after scaling up production. The continuous consumption of bioreactor bags, sensors, and tubing creates an invisible stream of operational expenditure.
When we shift our perspective from “purchase cost” to “total cost of ownership,” a different economic landscape unfolds.
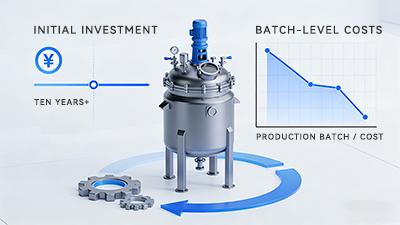
The core economic logic of stainless steel reactors lies in their complete lifecycle, where initial investment is amortized across every production batch over the next decade or longer. As production scale expands and operating time accumulates, per-batch costs decrease significantly, highlighting economies of scale.
More importantly, this model shifts the cost structure from ongoing dependence on external supply chains to internally controllable asset depreciation and maintenance. Its value lies not only in the present but also in the continuously diluted costs and sustained capacity dividends that accompany the enterprise's growth.
More importantly, this model shifts the cost structure from ongoing dependence on external supply chains to internally controllable asset depreciation and maintenance. Its value lies not only in the present but also in the continuously diluted costs and sustained capacity dividends that accompany the enterprise's growth.
Supply chain security has become an indispensable strategic element in modern biomanufacturing. Reliance on specific brands of disposable consumables means placing critical segments of production processes beyond corporate control. While global supply chains offer convenience, they also harbor risks of disruption and delays. When production schedules become deeply intertwined with distant factories' timetables and the smooth flow of logistics, autonomy over production is quietly relinquished.
Stainless steel bioreactors fundamentally eliminate absolute dependence on specific single-use consumables through proven CIP and SIP processes. This not only significantly reduces the risk of production downtime caused by consumable shortages or quality fluctuations but also restores core control and rhythm over the production process to the process team.
Meanwhile, its durability and standardized design also enable localized service, maintenance, and upgrades, further solidifying the resilience and independence of the production system. In an era where uncertainty has become the norm, anchoring the lifeline of production to an internally controllable system has become a strategic necessity.
Stainless steel bioreactors fundamentally eliminate absolute dependence on specific single-use consumables through proven CIP and SIP processes. This not only significantly reduces the risk of production downtime caused by consumable shortages or quality fluctuations but also restores core control and rhythm over the production process to the process team.
Meanwhile, its durability and standardized design also enable localized service, maintenance, and upgrades, further solidifying the resilience and independence of the production system. In an era where uncertainty has become the norm, anchoring the lifeline of production to an internally controllable system has become a strategic necessity.
As the biopharmaceutical industry advances into a new phase of high-quality, high-efficiency commercial manufacturing, the criteria for selecting production platforms are evolving. True “stability” is no longer synonymous with conservatism, but rather represents a higher level of certainty and flexibility chosen through insight into complexity. It signifies a capability—a technological pathway that provides a solid foundation for assured production—and a more mature, deliberate direction for industrial evolution.
Next time, we won't stop at concepts and promises. We will fully reveal how we fuse the three dimensions of “reliability,” “economy,” and “autonomy” into a single piece of equipment, making it the most dependable and productive cornerstone in your process landscape.
Stay tuned for the final chapter.
Next time, we won't stop at concepts and promises. We will fully reveal how we fuse the three dimensions of “reliability,” “economy,” and “autonomy” into a single piece of equipment, making it the most dependable and productive cornerstone in your process landscape.
Stay tuned for the final chapter.