
When we perform fermentation experiments, the pH of the fermentation broth is one of the important parameters of the fermentation process. This is because pH has an impact on the growth of microbial cells and the production of products (by-products).
In order to enable the microorganisms to flourish and grow in the most suitable ph range and eventually synthesize the target metabolites. We need to strictly control the ph value in the fermentation. Therefore, fast and accurate online monitoring of value changes and timely pH regulation becomes one of the key factors for the ultimate success or failure of fermentation.
How to regulate the pH value of the laboratory fermenter during the fermentation process?
In order to enable the microorganisms to flourish and grow in the most suitable ph range and eventually synthesize the target metabolites. We need to strictly control the ph value in the fermentation. Therefore, fast and accurate online monitoring of value changes and timely pH regulation becomes one of the key factors for the ultimate success or failure of fermentation.
How to regulate the pH value of the laboratory fermenter during the fermentation process?
Working principle of pH electrode
Firstly the pH electrode will continuously measure and determine the pH in the fermentation broth during the fermentation process.
pH measurements are part of a primary cell system, which serves to convert chemical energy into electrical energy.The voltage between the positive and negative terminals of a battery is called the electrode potential.And the electrode potential is composed of a measuring electrode (indicator electrode) and a reference electrode.
pH measurements are part of a primary cell system, which serves to convert chemical energy into electrical energy.The voltage between the positive and negative terminals of a battery is called the electrode potential.And the electrode potential is composed of a measuring electrode (indicator electrode) and a reference electrode.
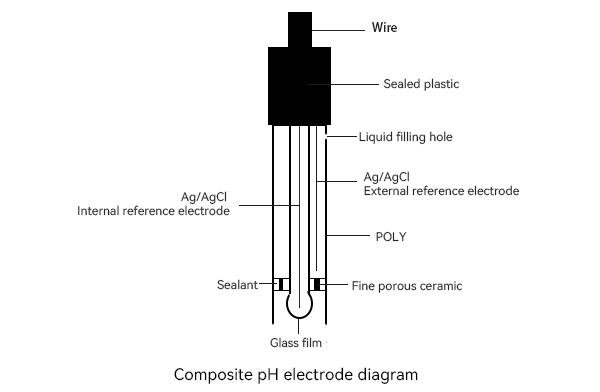
The pH electrode is based on the principle of the Nernst equation to measure acidity and alkalinity.The pH value is generally linearly related to the electric potential of the electrode and is usually calibrated with a standard buffer and the solution being measured.
The slope of the electrode response of pH, meaning that the pH electrode converts the electrode's millivolt (mV) signal into a pH value. Since the reference electrode potential is stable, this electric potential is related to the hydrogen ion activity of the measured solution, and eventually the pH value is obtained by comparing the voltage difference measured by the standard solution and dividing it by the standard buffer difference.
The slope of the electrode response of pH, meaning that the pH electrode converts the electrode's millivolt (mV) signal into a pH value. Since the reference electrode potential is stable, this electric potential is related to the hydrogen ion activity of the measured solution, and eventually the pH value is obtained by comparing the voltage difference measured by the standard solution and dividing it by the standard buffer difference.

Adjustment of fermenter pH value
Take HOLVES laboratory fermenter as an example, after the pH electrode has measured the value, the result will be fed directly to HF-Control V2.2 control system, and the system will adjust the replenishment and other operations according to the original set value to control the pH value in the tank in the best range.
The pH control mainly relies on ACID and BASE pumps for acid and alkaline working operations.
Peristaltic pumps are controlled by PID:
① When the pH value is higher than the set value, the system will automatically start the ACID pump to add acid until the set value is reached.
The pH control mainly relies on ACID and BASE pumps for acid and alkaline working operations.
Peristaltic pumps are controlled by PID:
① When the pH value is higher than the set value, the system will automatically start the ACID pump to add acid until the set value is reached.
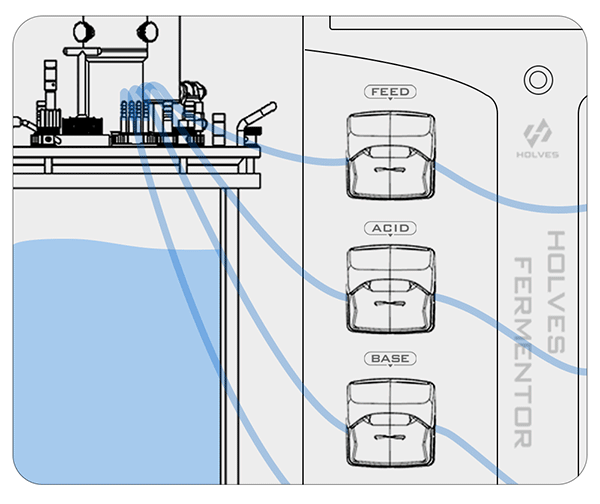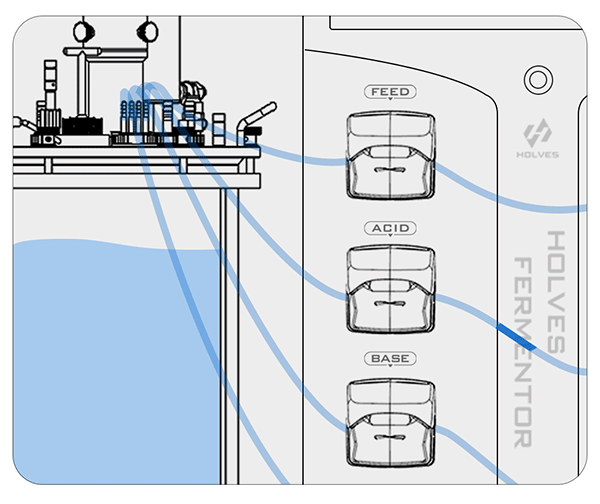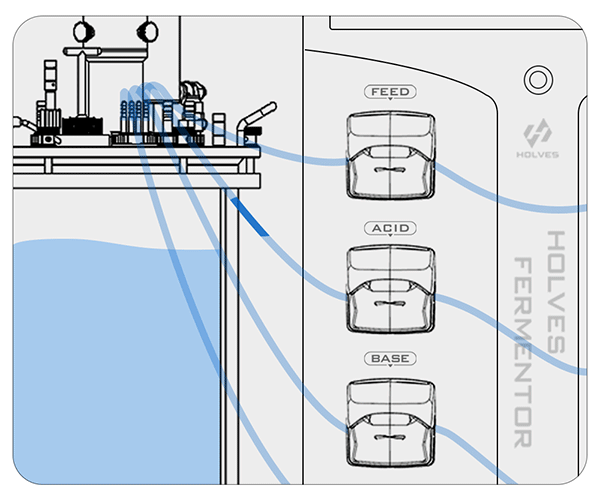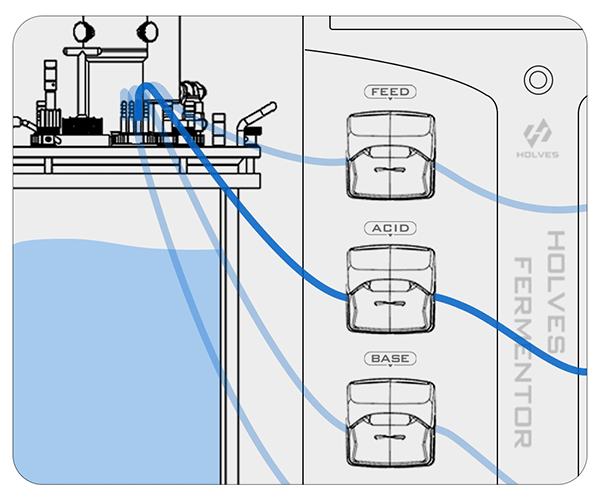
② On the contrary, when the pH value is less than the set value, the BASE pump will be activated for alkali addition adjustment.
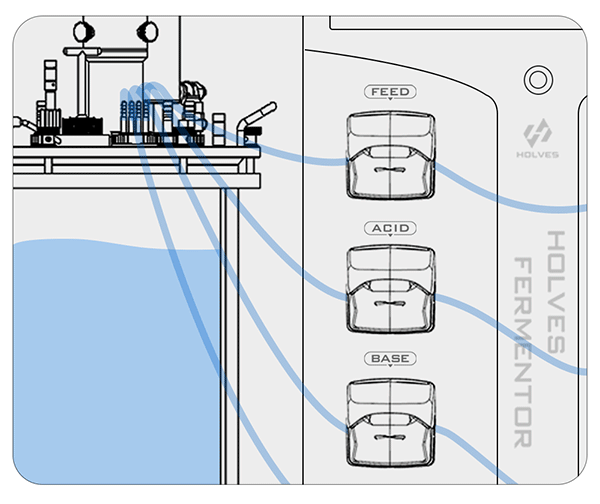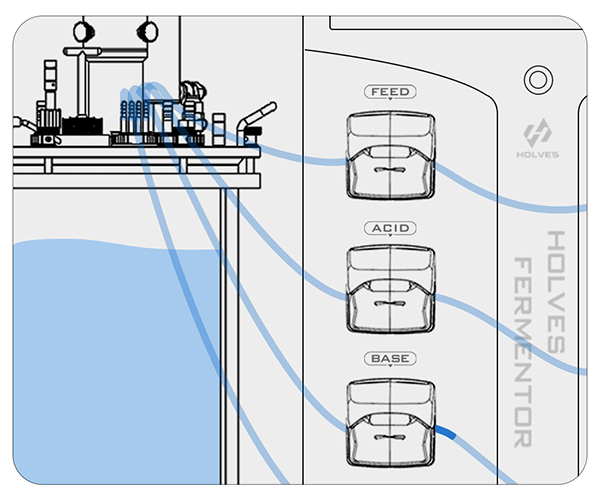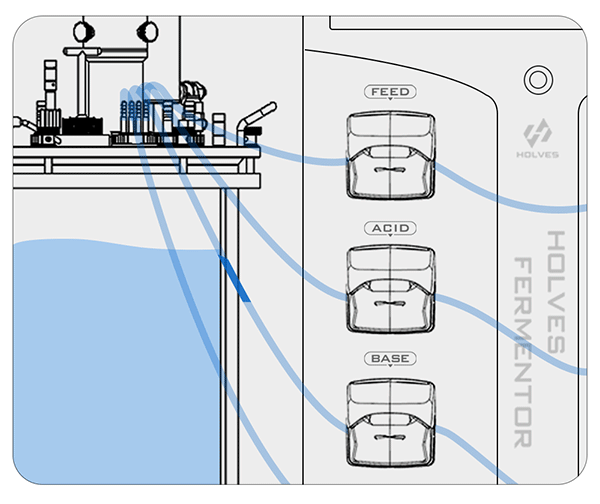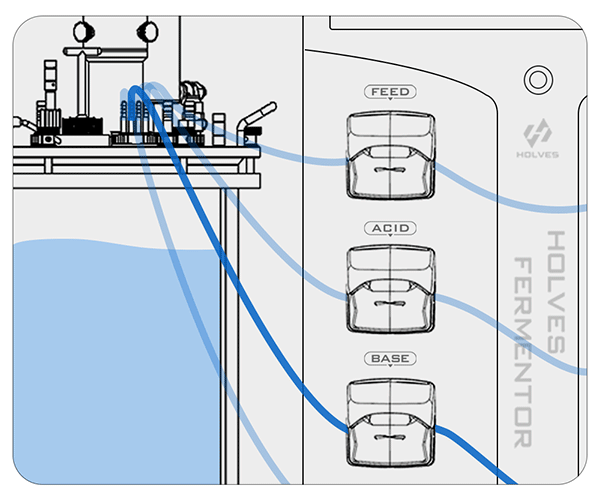
This process may be influenced by some factors and cause delays, so the closer the value is to the set value, the more the pump speed needs to slow down until the adjustment dead zone, because the dead zone means that the value can be freely adjusted.
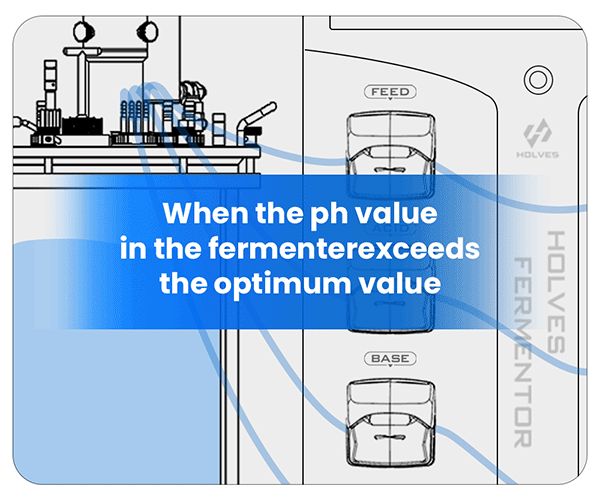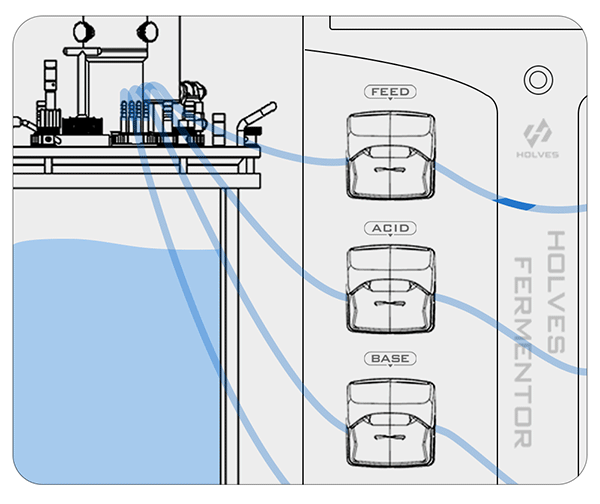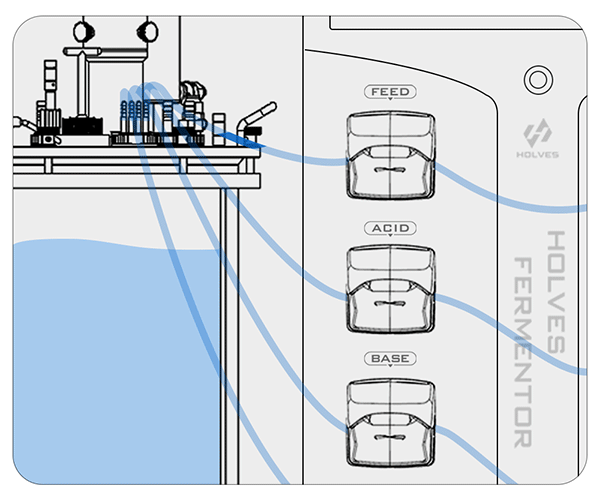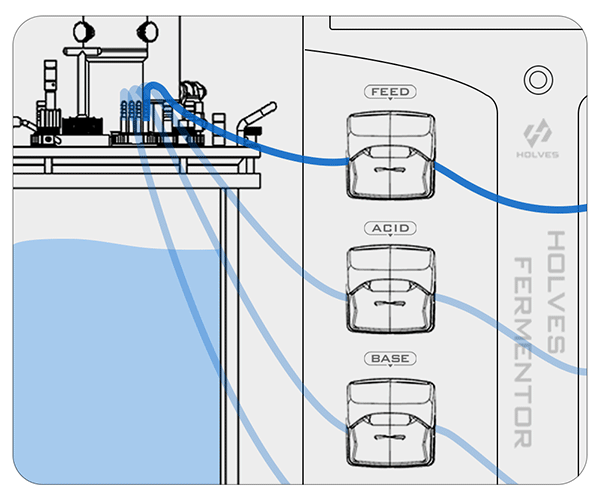
③ When the pH value in the fermenter exceeds the optimum value, it means that the condition of the flora is starved, and then the FEED pump can be activated for replenishment.
③ When the pH value in the fermenter exceeds the optimum value, it means that the condition of the flora is starved, and then the FEED pump can be activated for replenishment.
These are the contents of the laboratory fermenter in the fermentation process for pH adjustment, the next article will explain the principle of dissolved oxygen control.
Here is the Holves brand website, https://www.bjholves.com/. Providing different types of industry information, technical knowledge, and solutions, we have developed and produced several new laboratory fermenter, bioreactor, tangential flow filtration system and other equipment to meet your needs from experimental to industrial production.








