In the field of biomedicine, every technological leap brings new hope for human health. Recently, two top academic journals, “Nature” and “Cell”, have continuously reported the pioneering achievements of Prof. Xu Huji's team at Shanghai Changzheng Hospital in the field of general-purpose CAR-immunocyte therapy, which has attracted widespread attention in the industry.

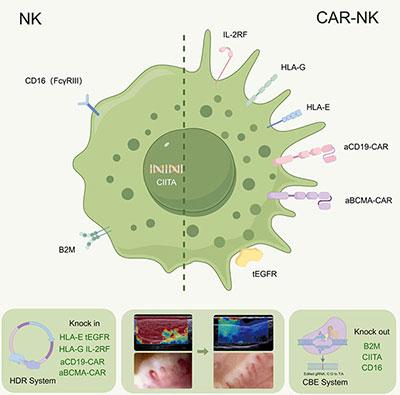
Prof. Xu's team has pioneered the development of universal CAR-NK cells using induced pluripotent stem cell (iPSc) technology for the treatment of refractory systemic sclerosis, and has achieved remarkable therapeutic results. This breakthrough not only provides a new “weapon” for cell therapy, but also takes a key step in solving the problems of limited donor source and high cost of traditional CAR-T cell therapy, which paves the way for industrialized production of cellular drugs and price stabilization.
Systemic sclerosis is a serious autoimmune disease with a mortality rate of over 40% within 10 years of onset. Traditional treatments can only provide temporary relief of symptoms and cannot address the root cause of the disease. Prof. Xu's team's CAR-NK cell therapy brings new hope for this disease. The therapy achieves significant relief from the disease by utilizing CAR-NK cells, a new type of immune cell therapy, to accurately and rapidly remove diseased B-cells from the patient's body.
In a clinical trial, a 36-year-old female patient treated with CAR-NK showed softer skin, improved cardiorespiratory function, and significant improvement in several indicators. This result demonstrates the significant efficacy of CAR-NK cells in the treatment of autoimmune diseases. Through the induced pluripotent stem cell (iPSc) technology, the unlimited “copying” of cells has been realized, which ensures the homogeneity and stability of cell quality, and provides a possibility for the industrial production of cellular drugs.
Systemic sclerosis is a serious autoimmune disease with a mortality rate of over 40% within 10 years of onset. Traditional treatments can only provide temporary relief of symptoms and cannot address the root cause of the disease. Prof. Xu's team's CAR-NK cell therapy brings new hope for this disease. The therapy achieves significant relief from the disease by utilizing CAR-NK cells, a new type of immune cell therapy, to accurately and rapidly remove diseased B-cells from the patient's body.
In a clinical trial, a 36-year-old female patient treated with CAR-NK showed softer skin, improved cardiorespiratory function, and significant improvement in several indicators. This result demonstrates the significant efficacy of CAR-NK cells in the treatment of autoimmune diseases. Through the induced pluripotent stem cell (iPSc) technology, the unlimited “copying” of cells has been realized, which ensures the homogeneity and stability of cell quality, and provides a possibility for the industrial production of cellular drugs.
HOLVES is deeply engaged in the field of biomanufacturing and has been committed to promoting technological progress and industrial upgrading. This breakthrough in CAR-NK cell therapy coincides with HOLVES' layout in the cell therapy field. In the article “Breakthroughs and Challenges in the Downstream Purification Process of Lentiviral Vectors” previously published by us, we have discussed in depth the optimization path of lentiviral vector purification technology, the core link of CAR-T therapy. The clinical success of generic CAR-NK cells further confirms that cell therapy technology is developing in the direction of scale-up, homogenization and low cost.
HOLVES always pays attention to the cutting-edge developments in the field of cell therapy and actively responds to the needs of the industry. In the purification of lentiviral vectors, we are constantly exploring key processes such as purification and concentration, in an effort to improve production efficiency and reduce costs. We look forward to exploring the infinite possibilities of biomanufacturing with industry colleagues, and helping more innovative cell therapy products move from the laboratory to the clinic for the benefit of patients.
HOLVES always pays attention to the cutting-edge developments in the field of cell therapy and actively responds to the needs of the industry. In the purification of lentiviral vectors, we are constantly exploring key processes such as purification and concentration, in an effort to improve production efficiency and reduce costs. We look forward to exploring the infinite possibilities of biomanufacturing with industry colleagues, and helping more innovative cell therapy products move from the laboratory to the clinic for the benefit of patients.